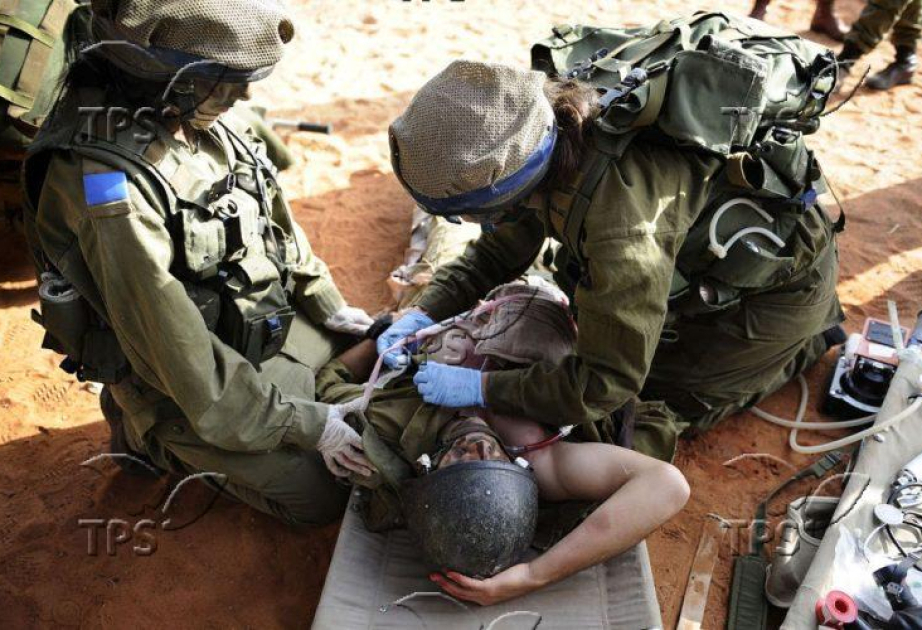
A groundbreaking discovery could dramatically change the way severe blood loss is treated in emergencies, according to The Press Service of Israel (TPS-IL).
By activating a specific protein shortly after bleeding begins, the team not only tripled survival rates but also showed, for the first time, that it is possible to preserve vital organ function at the cellular energy level during hemorrhagic shock.
Hemorrhagic shock, caused by severe blood loss, remains the leading cause of preventable death in trauma cases worldwide. Until now, no treatment had demonstrated such a profound improvement in survival while simultaneously protecting organs from energy depletion and damage.
But a joint project between the Hebrew University-Hadassah Medical School and the Medical Corps of the Israel Defense Forces found that activating a protein called PKC-ε strengthened the body’s resilience. The research was recently published in the peer-reviewed journal Scientific Reports. The study was led by Dr. Ariel Furer and Dr. Maya Simchoni from the Institute for Research in Military Medicine.
“Massive hemorrhage remains one of the most critical challenges faced in emergency medicine, particularly in battlefield and civilian trauma scenarios,” said Furer. “Our findings suggest that activating PKC-ε can be a highly effective therapeutic approach, not only improving survival but also preserving organ function by maintaining cellular energy production during extreme trauma.”
In a controlled experiment using a porcine model, researchers induced hemorrhagic shock by withdrawing 35% of the animals’ total blood volume. Five minutes after bleeding began, some animals were treated with a PKC-ε activator peptide. The results were striking: 73% of treated animals survived, compared to just 25% of those that received no treatment.
Treated animals also maintained stronger cardiovascular stability, including better blood pressure, heart rate, and cardiac output—critical factors in surviving massive trauma.
Crucially, the researchers found that activating PKC-ε enhanced mitochondrial function in heart tissues, preserving the cells’ ability to produce energy under extreme stress. This finding is significant because it suggests that the treatment does more than stabilize vital signs—it protects the body’s organs at a fundamental cellular level, offering a new way to prevent the catastrophic organ failures often seen after major blood loss.
Current treatments for hemorrhagic shock typically rely on fluid resuscitation, which can sometimes worsen injury by triggering ischemic-reperfusion damage. Ischemic-reperfusion damage happens when blood supply returns to tissue after a period of lack of oxygen and nutrients due to restricted blood flow.
In contrast, activating PKC-ε offers a novel strategy that strengthens the body’s internal resilience without the harmful side effects of traditional methods.
Furer stressed that additional research will be essential to confirm the treatment’s effectiveness in human clinical settings.
Beyond the laboratory, the potential applications are wide-ranging. PKC-ε activation could be used by first responders, military medics, and emergency physicians to improve survival in trauma victims before they reach full medical care. It could also play a critical role in disaster response scenarios and help prevent organ failure in critically injured patients.
“Our findings open new avenues for targeted therapeutic strategies that can be administered by first responders in emergency settings, potentially saving countless lives worldwide,” he said. “Future clinical trials will be critical to move from promising laboratory results to practical, life-saving applications.”